In Portugal, statistics show that around 800 thousand people suffer from chronic kidney disease and 18,000 patients are being treated (two thirds on dialysis and one third transplanted), with primary prevention being fundamental to reduce the number of people suffering from this disease1.
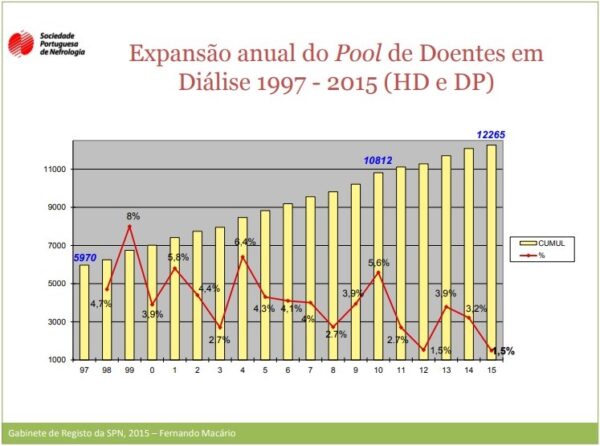
Figure 1 – Annual Expansion of the Dialysis Patient Pool 1997 – 2015 [Report of the Portuguese Society of Nephrology Registry Office, , 2015]
Dialysis is a catabolic process that leads to malnutrition and that all stages of chronic kidney disease (CKD), or chronic renal failure (CRF) as it is usually known, and DRT compel the assessment, monitoring and implementation of specific diets.
Also nephrotic syndrome (proteinuria higher than 3.5 g/day) requires specific restrictions and diets.
Thus, healthy nutrition represents an obligatory aid in the management of chronic kidney disease and regardless of the state of renal function, it should be an essential part of prevention and treatment, being able to:
- Guarantee nutritional needs, avoiding undernutrition or malnutrition.
- Help maintain adequate weight.
- Guarantee energy needs according to activity and physiological state.
- Reduce weight on renal function, avoiding degeneration.
- Reduce symptomatology such as nausea and/or dysgeusia.
- Prevent myolysis.
- Assist in the control of glucose and/or diabetes and other metabolic syndromes.
We suggest:
- Substitution of milk with vegetable drinks such as oat or soya beverages
- Ground flaxseed
- Glutathione
- Probiotics
- Cordyceps sinensis
- Cupressus sempervirens L.
- Fragaria vesca
- Phyllanthus spp.
- Solidago virgaurea L.
Actual Renal Failure
Acute renal failure (ARF) has a sudden onset and rapid deterioration of renal function and a high mortality rate (around 60-65%), however, it can be reversed, unlike CRD. Of course, in the absence of adequate medical and nutritional follow-up, progression to chronic kidney disease is high risk and with it, the need for dialysis and/or transplantation.
NutritionalI ntervention and Treatment
In certain patients, short-term dialysis may be necessary to avoid renal injury and recover basal function, including diuretics, potassium chelating resins that act at the intestinal level, insulin when hyperglycaemia occurs, and bicarbonates in patients with acidosis. Since AKI is generally a catabolic process, careful nutritional intervention is necessary and common guidelines in hospital practice:
- Attenuate the catabolism of AKI to avoid visceral and somatic protein loss, being that the protein requirement will vary according to renal status, dialysis and protein status.
- On dialysis or if renal function is increased: 1.2 to 1.3g/kg body weight
- Without dialysis: 0.6 – 0.8g/kg body weight
- Ensure calorie intake to avoid energy loss through protein
- Sensibly 35kcal/kg body weight
- Possible limitation of electrolytes
- Maximum sodium [2 – 3]g/day to avoid hypertension and fluid retention
- Monitor potassium and phosphorus levels
- Monitor fluid balance
- On dialysis: intake should be kept between 1 and 1.5L/day
- Without dialysis: restriction usually necessary to equalise urine output volume +500ml
Chronic Kidney Disease
In chronic kidney disease (CKD) there is a gradual and progressive loss of kidney function, which is irreversible. The most common causes are diabetes and hypertension, which cause about 69% of cases, and may be of infectious origin, exposure to nephrotoxic substances or immune diseases.
The patient may lose up to 75% of renal function and remain asymptomatic and it is necessary to use the glomerular filtration rate (GFR) to verify the state and dysfunction of renal function and, consequently, the stage of CRD. When the GFR reaches values below 15 ml / min / 1.72m2 the patient enters stage 5 and is classified as pre-dialysed or non-dialysed, as this distinction will vary the nutritional intervention which varies according to the stage of CRD and the need for dialysis.
Non-dialysed CRD
As GFR decreases, the kidneys lose the ability to maintain acid-base and water-electrolyte balance, and the patient must be followed up with complementary diagnostic tests including haemoglobin, haematocrit, calcium, phosphorus, lipid profile and, of course, plasma urea and creatinine, potassium, carbon dioxide and chlorides.
In this stage, the most important thing is to preserve renal function for as long as possible, postponing as much as possible the entry into stage 5 and thus dialysis. It is therefore important to monitor/restrict protein and electrolytes (especially potassium).
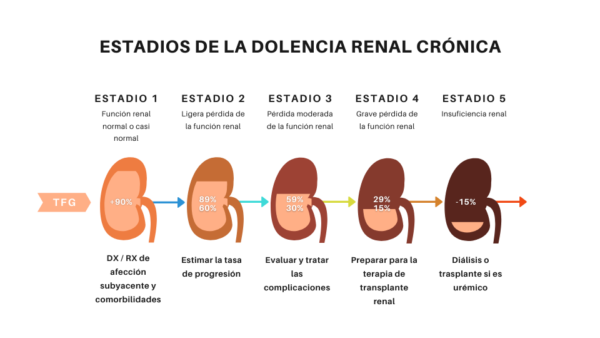 Figure 2 – Stages of Chronic Kidney Disease
Figure 2 – Stages of Chronic Kidney Disease
Nutritional Intervention and Treatment
In clinical terms, interventions target the main renal functions which in these patients are in rapid decline and use phosphorus chelators, intravenous iron, parathyroid hormone (PTH) suppressing agents, which are adjusted according to the results of follow-up laboratory tests.
The Kidney Disease Outcomes Quality Initiative (KDOQI) guidelines establish practical recommendations based on clinical evidence according to the stage of CRD, recommending nutritional assessment and monitoring from stage 2 onwards to prevent further damage to renal function, with control of blood glucose and hypertension being of extraordinary importance. Direct nutritional intervention should help control uraemic symptoms such as diarrhoea, vomiting and anorexia and prevent malnutrition. Specific indications include:
- Calorie and protein calculation based on standardised body weight (BW) or adjusted body weight (BW) with maintenance needs only, and caloric intake should be sufficient to meet caloric needs and avoid protein catabolism.
- Special attention should be given to the fact that stress and activity level change the needs and high biological value (HBV) proteins should be chosen, which should be at least 50% of the protein intake.
- In the case of a patient on diuretics, potassium intake will not need to be limited at first, but will be implemented as it rises above normal serum levels.
- Sodium restriction below 1g.
- Control of phosphorus intake (through which it is also possible to limit calcium intake) to control serum PTH levels, with the need for drugs that inhibit intestinal absorption of phosphorus.
- Limit calcium intake to a maximum of 2g/day.
- Limit fluid intake, especially if congestive heart failure is present and that patients treated with diuretics should be especially monitored, as these may cause progression of renal disease due to volume depletion.
DRC Dialysis
When the patient reaches stage 5, the need for dialysis arises, which acts as an artificial filtration of the blood. There are 2 main types of dialysis: haemodialysis and peritoneal dialysis. In haemodialysis, blood from an artery circulates through a mechanical dialyser where it is filtered and then returned to the bloodstream through a parallel vein. In peritoneal dialysis, the patient’s own peritoneum acts as a filtering membrane, with the dialysate (hyperosmolar solution) entering through a catheter that passes through the abdominal wall and is infused into the peritoneal cavity. In both situations, the diet is less restrictive compared to the pre-dialysis state.
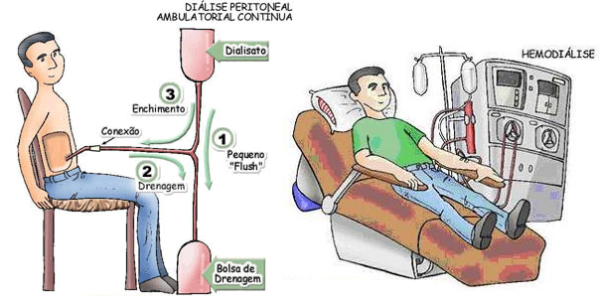
Figure 3 – Haemodialysis and Peritoneal Dialysis
Nutritional Intervention and Treatment
Calorie and protein requirements should be based on PCP or PCA and provide only amounts needed for maintenance. Again, periods of stress and high physical activity should increase requirements and therefore intake. As the dialysis process increases protein loss, it may be necessary to increase intake (compared to the non-dialysed phase of the disease). Preference should be given to AVB proteins by at least 50%.
Since peritoneal dialysis uses glucose in its dialysate, in these patients it is especially important to monitor blood glucose and caloric intake, and the diabetic patient should be followed closely. Other monitoring needs are:
- Potassium
- Peritoneal dialysis: as this procedure may increase the diuresis of this electrolyte, these patients may require supplementation.
- Haemodialysis: if leaks are present (common in the possible aetiologies of CRD such as polycystic kidney disease and significant residual renal function) or diuretics are used, supplementation may be necessary.
- Sodium
- Intake should be limited to control thirst and thus keep fluid intake restricted, avoiding oedema.
- Phosphorus
- Limit intake to reduce PTH stimulation, chelating drugs inhibiting intestinal absorption may be necessary.
- Calcium
- Intake should be limited to doses of 2000mg/day (diet and possible supplementation). Calcium-based phosphorus chelators, commonly used in the food industry, should be carefully monitored.
To conclude, the correct appetite and dietary intake must be ensured, and it is preferable that the diet be free rather than placing too many restrictions leading to undernutrition.
In certain patients, where intake is poor, specific supplements such as appetite stimulants and liquid supplements can be used.
Nutritional Management of Chronic Kidney2
As chronic kidney disease (CKD) progresses, uraemia increases, there is an electrolyte imbalance, acid-base balance and water retention. In this picture, bone disease is common and protein-energy wasting is also common.
Thus, the authors of the latest review on nutrition in CKD published in November 2017, which highlights important areas of nutritional management, advocate that a specific diet in these patients can improve the clinical condition, and recommend nutritional and dietary doses of protein, sodium, potassium, phosphorus, calcium, fibre, calories and fat according to the stage of the disease.
According to this review bicarbonate excretion in healthy subjects is approximately 30-34.6 ± 12.3 mmol/L, however affected patients excrete higher values, possibly due to incomplete oxidation of lipids and carbohydrates leading to increased production of lactic acid and ketoacids and consequently increasing the hydrogen ion load of daily acid production.
In patients at high risk of renal disease, they suggest a maximum of 1g/kg body weight, [0.6 – 0.8] g/kg body weight in patients with advanced stages of disease and/or elevated proteinuria, including here patients who will start dialysis.
In dialysis, they argue that if the patient performs 3 or more sessions per week and has residual renal function, the recommended protein doses should be higher than 1g/kg body weight, justifying that this is, according to a study with a duration of 3 years and 30,075 participants3, the one with the lowest mortality rate.
Therefore, they argue that a low-protein diet can reduce the progression of the disease and help patients avoid or increase the time without the need for dialysis, without the risk of changes in the mechanism of obtaining energy through protein or cachexia, to the consumption of vegetable protein, referring that this is associated with a better general state of health, being able to reduce intraglomerular pressure and proteinuria as well as reduce urea production.
In this regard, he even refers that severe restrictions on potassium intake should be avoided, as many potassium-rich foods, such as fresh fruits and vegetables, are rich in fibre and vitamins, have low acidogenesis and are healthier in cardiological terms as well as less atherogenic than low-potassium foods.
It concludes by stating that ‘given the high incidence and prevalence of chronic kidney disease and an urgent need for alternative strategies in disease management, nutritional interventions with pathology-specific beacons that are patient-targeted and cost-effective can help longevity and decrease the need for dialysis’.
Probiotics and Kidney Disease
Several studies defend the importance and demonstrate the impact of the microbiome on the state of health and well-being, protecting against pathogens, educating cells and contributing to various metabolic functions, directly or indirectly affecting various physiological functions4, showing that alterations in the composition and structure of the human microbiota, known as dysbiosis, can predispose to various pathologies, also explaining susceptibilities or resistance to certain diseases5.
In fact, these alterations to the microbiome are increasingly linked to the development of various diseases such as obesity6, cancer disease7, diabetes8, inflammatory bowel disease9, asthma10, cardiovascular disease11 and kidney disease2.
In fact, dysbiosis in CKD patients may contribute to an increase in toxic urea levels, which contributes to disease progression13.
It should be noted that interactions between the microbiome and renal disease are not unidirectional and therefore renal pathology affects the structure of the microbiota and contributes to dysbiosis.
Given that renal disease is associated with decreased fibre intake14 and increased frequency of antibiotic intake15, decreased intestinal transit, congestion and oedema of the gut walls, hyperacidosis and iron supplementation16, and that these factors are associated with dysbiosis and increased numbers of microbial pathogens in the gut, supplementation with the correct probiotics should be of paramount importance.
Many of these factors mentioned above affect intestinal permeability which is increased, leading to translocation of bacterial products across intestinal barriers and consequently triggering immune responses that may explain the systemic inflammation that contributes negatively to the development of cardiovascular and renal disease17.
Another possible mechanism for dysbiosis in renal patients results from increased gastrointestinal urea secretion18 that being hydrolysed by microorganisms leads to elevated ammonium formation which affects commensal bacteria and causes imbalance in the gut microbiota.
| Details | |
| Theme | Nutrition in Renal Pathology
|
| Nutrition | Low protein diet preferably of plant origin.
Monitor and limit fluid, potassium, calcium, phosphorus and sodium intake. Suggest increased intake of garlic, cinnamon, Zingiber officinale, Camellia sinensis and Curcuma longa. |
| Phytotherapy / Nutrient | Vegetable oat or soya drinks.
Ground flaxseed Glutathione, Probiotics; Cordyceps sinensis; Cupressus sempervirens L.; Fragaria vesca; Phyllanthus spp.; Solidago virgaurea L. |
| Lifestyle habits | Moderate physical exercise, preferably hydrogymnastics; sleep hygiene; dietary hygiene.
|
| Products with beneficial action | Renes Forte, Probiotic5, Advanced Pro, Turmeric Max Liposomado, Magnesium Citrate Liposomado, Omega 3 MaxPower.
|
| Link’s | UNC Kidney Health Library; An Integrative Approach to Advanced Kidney Disease in the Elderly; Guidelines for the use of alternative medicine for kidney patients
|
| Keyword’s | #Rim #Naturopatia #Nutrición #Dolencia_Renal_Crónica #Probióticos #Glutatión #Probióticos #Cordyceps_sinensis #Cupressus_sempervirens #Fragaria_vesca #Phyllanthus #Solidago_virgaurea
|
BIBLIOGRAPHY & SCIENTIFIC EVIDENCE STUDIES
[1] Portal de Diálisis – Dia Mundial del Riñón 2017 in https://www.portaldadialise.com/articles/dia-mundial-do-rim-2017
The Clinical Dietitians’s Essential Pocket Guide, 1st Edition; Koogan, 2009;
[2] Nutritional Management of Chronic Kidney Disease. N Engl J Med. 2017 Nov 2;377(18):1765-1776.
[3] Is controlling phosphorus by decreasing dietary protein intake beneficial or harmful in persons with chronic kidney disease? Am J Clin Nutr. 2008 Dec;88(6):1511-8.
[4] How host-microbial interactions shape the nutrient environment of the mammalian intestine. Annu Rev Nutr. 2002;22:283-307.
Host-bacterial mutualism in the human intestine. Science. 2005 Mar 25;307(5717):1915-20.
[5] Dysbiosis of the gut microbiota in disease. Microb Ecol Health Dis. 2015 Feb 2;26:26191.
[6] Obesity and the gut microbiome: pathophysiological aspects. Horm Mol Biol Clin Investig. 2014 Jan;17(1):53-61.
[7] The microbiome and obesity-an established risk for certain types of cancer. Cancer J. 2014;20:176–180.
Microbiome, inflammation, and cancer. Cancer J. 2014;20:181–189.
[8] The intestinal microbiome in type 1 diabetes. Clin Exp Immunol. 2014;177:30–37.
Does the gut microbiome hold clues to obesity and diabetes? Curr Biol. 2013;23:R359–362.
[9] Irritable bowel syndrome, inflammatory bowel disease and the microbiome. Curr Opin Endocrinol Diabetes Obes. 2014;21:15–21.
The microbiome in inflammatory bowel disease: current status and the future ahead. Gastroenterology. 2014;146:1489–1499.
[10] Microbiota in allergy and asthma and the emerging relationship with the gut microbiome. Cell Host Microbe. 2015;17:592–602.
[11] Elevated levels of circulating DNA in cardiovascular disease patients: metagenomic profiling of microbiome in the circulation. PLoS One. 2014 Aug 18;9(8):e105221.
Contrasting circulating microbiome in cardiovascular disease patients and healthy individuals. Int J Cardiol. 2013;168:5118–5120.
[12] The intestinal microbiota, a leaky gut, and abnormal immunity in kidney disease. Kidney Int. 2013;83:1010–1016.
[13] Gut microbiome and kidney disease: a bidirectional relationship. Pediatr Nephrol. 2017 Jun;32(6):921-931.
[14] Food intake characteristics of hemodialysis patients as obtained by food frequency questionnaire. J Ren Nutr. 2002;12:17–31.
[15] Short-term antibiotic treatment has differing long-term impacts on the human throat and gut microbiome. PLoS One. 2010 Mar 24;5(3):e9836.
[16] Dietary management of chronic kidney disease patients: protein-restricted diets supplemented with keto/amino acids. Am J Nephrol. 2005;25 Suppl 1:1-28.
[Chronic kidney disease (CKD)–recent progress. Topics: VII. Management of chronic kidney disease (CKD) and treatment; 1. Dietary treatment and life-style management in CKD patients]. Nihon Naika Gakkai Zasshi. 2012 May 10;101(5):1340-6.
Dietary trends and management of hyperphosphatemia among patients with chronic kidney disease: an international survey of renal care professionals. J Ren Nutr. 2014 Mar;24(2):110-5.
[17] Gut microbiome in chronic kidney disease. Exp Physiol. 2016 Apr;101(4):471-7.
[18] The gastrointestinal tract in uremia. J Assoc Physicians India. 1997 Nov;45(11):833-4.